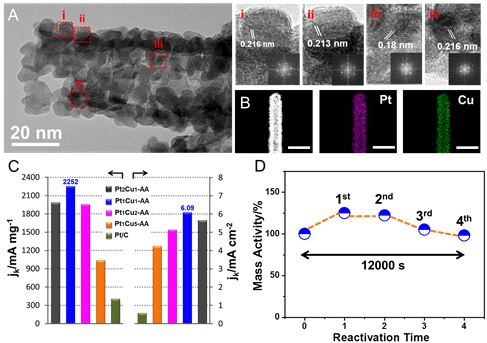
Recently, the research team of Professor Yu Shuhong from the Hefei National Laboratory for Physical Sciences at the Microscale and the School of Chemistry and Materials Science at our university has successfully prepared a one-dimensional ultrafine four-component PtPdRuTe nanotube and a one-dimensional open-celled PtCu nanotube catalyst. The catalyst has stable and efficient methanol oxidation performance. Relevant achievements were respectively titled "Synthesis of Low Pt-Based Quaternary PtPdRuTe Nanotubes with Optimized Incorporation of Pd for Enhanced Electrocatalytic Activity" and "Highly crystalline PtCu nanotubes with three dimensional molecular accessible and restructured surface for efficient catalysis" and were published in J.Am. Chem.Soc. and Energy Environ.Sci. The direct methanol fuel cell is a proton exchange membrane fuel cell with methanol as a liquid fuel, which not only has advantages of rich fuel source, low cost, convenient storage and transportation, and the like, but also has a high energy density and has received extensive attention. However, the development of methanol fuel cells is hampered by the slow reaction kinetics of the methanol reaction at the anode and the vulnerability of metal platinum catalysts to poisoning and the need to increase platinum loading. Therefore, the number of active sites exposed to the catalyst and the surface structure, composition, and atomic arrangement are critical for improving platinum utilization and catalytic performance. At present, a large number of studies have focused on exploring the formation of alloys or heterostructure catalysts with different transition metals and platinum to modify the platinum electronic structure and achieve the goal of reducing platinum loading and increasing platinum utilization. Nanostructured materials such as nano cages, nano-frames, hollow spheres, etc., also have an active surface that allows the catalyst to have a high specific surface area, porosity, and three-dimensional contact with the reactants, maximizing the use of active metals to reduce costs. In addition, another important key issue that restricts the development of fuel cells is the stability of the catalyst. At present, in the harsh electrochemical environment, the performance improvement of many platinum-based catalysts is still limited by the composition or structural stability of nanomaterials. During electrochemical testing, platinum tends to oxidize or dissolve resulting in a loss of active specific surface area or a decrease in catalytic activity, which is related to the structure and composition of the nanomaterial. Concerning the constraints of the development of fuel cells, the properties and electronic structures of platinum-based nanomaterials are improved through the adjustment of the structure, composition, surface, bond length and other parameters of the catalysts, and the study of the improvement of its intrinsic activity and the mechanism for maintaining the stability is particularly important. important. The key question is how to achieve the controllable composition of multi-element catalysts by using simple and effective preparation methods, and to effectively use the advantages and effects of different atoms in catalytic reactions, and how to design highly efficient and stable anode catalysts with reproducible catalytic properties. The practical application of methanol fuel cells has an important role in promoting. Figure 1. HAADF/STEM and HRTEM images of (AC) Pt17Pd16Ru22Te45 nanotubes; (D) Mass activity of PtPdRuTe, PtRuTe, and Pt/C catalysts normalized to the loading mass of PtPd and Pt, respectively; (E) Pt17Pd16Ru22Te45 nanometers. Tube CV curve before and after 1000 cycles. For this purpose, the researchers used a highly reactive ruthenium nanowire as a template, and used a potential difference to perform the displacement and substitution reaction, and successfully prepared a quaternary PtPdRuTe nanotube catalyst with controllable composition, using palladium with a high potential potential. Platinum forms an alloy and achieves controllable composition, which is important for reducing the platinum oxidation potential to enhance platinum stability. As a four-way catalyst, each element plays an indispensable role in catalytic applications: while the germanium nanowire acts as a reducing agent and template, the unsubstituted germanium atoms together with the other three precious metal elements support the nanotube structural framework. And reduces the composition of the precious metal; the platinum atom provides the dissociated active site of methanol to form Pt-CO, while the hydrazine atom provides the dissociating water to form the active site of Ru-OH, and promotes the oxidation of CO on the platinum active site to form CO2; moderate amount of palladium atom While the addition of (16%) has a modifying effect on the platinum electronic structure, it is more important that palladium can increase the oxidation potential of platinum to reduce the oxidation and etching of platinum atoms and enhance the compositional stability of the catalyst. By regulating the composition, the interaction between atoms is enhanced, and the advantages of different atoms in the catalytic reaction are exerted, thereby promoting the efficient catalytic reaction (see FIG. 1). This research result was published on J.Am.Chem.Soc. (J.Am.Chem.Soc. 2017, 139, 5890-5895), and the co-first author of the dissertation is Ph.D. student Ma Sie and associate researcher Li Huihui. Figure 2. (A) HRTEM images of PtCu nanotubes composed of highly crystalline nanoparticles. (i-iv) is a magnified HRTEM image of the area indicated by boxes i, ii, iii, iv in (A); (B) STEM images of nanotubes and high resolution STEM-EDS elemental maps showing Pt and Cu elements Uniformly distributed, the scale in the figure is 50 nm; (C) Comparison of mass-to-activity and area-specific activity of the relevant catalyst; (D) Activity of mass-specific activity as a function of regeneration time. In addition to the stability maintenance of the catalyst, in the face of limited and expensive platinum resources, active regeneration or repeated use of the catalyst is also an important issue. To address this issue, the researchers demonstrated the high activity and long-term stability of one-dimensional open-celled PtCu nanotubes in methanol oxidation catalysis. The mass specific activity and area specific activity of Pt1Cu1-AA nanotubes were 2252 mA mg-1 and 6.09 mA cm-2, respectively, 5.5 and 10.3 times that of the commercial Pt/C catalyst. The activity of the nanotube catalyst after the stability test not only does not decrease, but is higher or maintains the initial value. Through a simple cyclic voltammetry test, a highly active regeneration of the catalyst can be achieved (Fig. 2). The stability is mainly due to the reconstitution of the catalyst surface during the activation process. The copper atoms on the surface are etched and the platinum atoms are rearranged to form a core-shell structure. The compressive stress increases and the stability of the catalyst is greatly improved. Combined with electron microscopy characterization results, after the stability test, the catalyst shows better crystallinity and maintains one-dimensional nanotube structure, and has good structural stability. Overall, the development of PtCu catalysts based on one-dimensional open-cell structures is expected to expand on other platinum-based materials. High-activity regeneration reduces the amount of platinum-based catalysts used in practical fuel cell applications. It is the study of catalyst stability. Provides new ideas. This research result was published on Energy Environ.Sci. (Energy Environ.Sci.2017, DOI: 10.1039/C7EE00573C). The co-first author of the paper is associate professor Li Huihui and doctoral student Fu Qiqi. The above research was supported by the National Natural Science Foundation of China Innovation Research Group, the National Natural Science Foundation of China, the Frontier Science Research Project of the Chinese Academy of Sciences, the National Major Scientific Research Project, the Suzhou Nanometer Science and Technology Collaborative Innovation Center, the Chinese Academy of Sciences Nanoscience Center of Excellence, and the Hefei University of Science Funding for the Center for Excellence in User Funding.
Mactech SMT
equipment is a collection of SMT production line solutions. MT SMT equipment
provides different solutions for different SMT production line process
requirements. MT equipment is made from durable and branded parts to provide
best and effective results. For more information regarding SMT equipment, you
can contact us well provide all the details you need. You may also visit our
official website to check for product catalogs and technical information about
our products.
SMT Equipment,SMT Placement Equipment,SMT Conveyor Equipment,SMT Peripheral Equipment ShenZhen KDW Electronics Co.,Ltd , https://www.smtsplicetape.com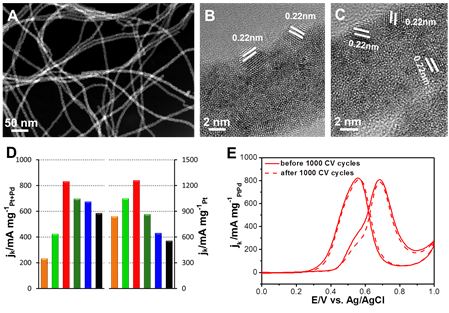
China University of Science and Technology takes a one-dimensional high-efficiency nanocatalyst for methanol oxidation reaction